Peryodat
Peryodat /pəˈraɪ.ədeɪt/ - yod va kisloroddan tashkil topgan anion. Bu yodning bir qator oksianionlaridan biridir va yod +7 oksidlanish darajasida mavjud bo'lgan seriyadagi eng yuqorisidir. Boshqa perhalogenatlardan, masalan, perxloratdan farqli o'laroq, u ikki shaklda bo'lishi mumkin: metaperyodat va ortoperyodat. Shu nuqtai nazardan, u qo'shni guruhdagi tellurat ioni bilan taqqoslanadi. U bir qator qarshi ionlar bilan birlashib, davriy kislota tuzlari sifatida ham qaralishi mumkin bo'lgan peryodatlar hosil qilishi mumkin.
Peryodatlar Geynrix Gustav Magnus va CF Ammermyuller tomonidan kashf etilgan; 1833-yilda birinchi marta davriy kislotani kim sintez qilgan[1]
Sintez
[tahrir | manbasini tahrirlash]Klassik usulda periodat ko'pincha natriy vodorod periodat (Na 3 H 2 IO 6) shaklida ishlab chiqarilgan.[2] Bu sotuvda mavjud, ammo yoodatlarning xlor va natriy gidroksid bilan oksidlanishi orqali ham ishlab chiqarilishi mumkin.[3] Yoki xuddi shunday, yodidlardan brom va natriy gidroksid bilan oksidlanish orqali:
- NaIO <sub id="mwLQ">3</sub> + Cl 2 + 4 NaOH → Na 3 H 2 IO 6 + 2 NaCl + H 2 O
- NaI + 4 Br 2 + 10 NaOH → Na 3 H 2 IO 6 + 8 NaBr + 4 H 2 O
Zamonaviy sanoat miqyosidagi ishlab chiqarish quyidagi standart elektrod potentsialiga ega bo'lgan PbO <sub id="mwQg">2</sub> anodida yodatlarning elektrokimyoviy oksidlanishini o'z ichiga oladi:
Metaperiodatlar odatda natriy vodorod periodatini nitrat kislota[2] bilan suvsizlantirish yoki ortoperiod kislotasini 100 ga qizdirish orqali suvsizlantirish orqali tayyorlanadi. °C vakuum ostida.
- Na 3 H 2 IO 6 + 2 HNO 3 → NaIO 4 + 2 NaNO 3 + 2 H 2 O
- H 5 IO 6 → HIO 4 + 2 H 2 O
Ular, shuningdek, gipoxloritlar kabi boshqa kuchli oksidlovchi moddalar bilan ishlov berish orqali to'g'ridan-to'g'ri yodlardan hosil bo'lishi mumkin:
- NaIO 3 + NaOCl → NaIO 4 + NaCl
Shakllar va o'zaro konversiya
[tahrir | manbasini tahrirlash]Peryodat suvli muhitda turli shakllarda bo'lishi mumkin, bunda pH nazorat qiluvchi omil hisoblanadi. Ortoperyodatda bir qator kislotali dissosiatsiya konstantalari mavjud.[5][6]
Orto- va metaperyodat shakllari ham muvozanatda mavjud.
- H4IO−6 ⇌ IO−4 + 2 H2O, K = 29
Shuning uchun ortoperyodat ba'zan metaperyodatning digidrati deb ataladi,[7] yozma ammo, H 5 IO 6 ning rentgen kristallografiyasi 5 ta ekvivalent I-OH guruhini ko'rsatganligi sababli, bu tavsif mutlaqo to'g'ri emas.[8]
Haddan tashqari pH darajasida qo'shimcha turlar paydo bo'lishi mumkin. Asosiy sharoitlarda diperyodat (ba'zan mezoperyodat deb ataladi) hosil qilish uchun suvsizlanish reaksiyasi sodir bo'lishi mumkin.
- 2 H</br> H IO 2−</br> H ⇌ H</br> H I</br> H O 4−</br> H + 2 H 2 O, K = 820
Kuchli kislotali sharoitda davriy kislota protonlanishi mumkin, bu ortoperyodat kationini beradi.[9]
- H6IO+6 ⇌ H5IO6 + H+, pKa = −0.8
Tuzilishi va bog'lanishi
[tahrir | manbasini tahrirlash]Orto- va metaperyodatda ham yod gipervalentdir, chunki u klassik ruxsat etilganidan ko'ra ko'proq bog'lanish hosil qiladi. Bu molekulalarda qo'sh bog'lanish yo'qligini tasdiqlovchi dativ aloqalar nuqtai nazaridan tushuntirildi.[10]
Aniq tuzilmalar qarama-qarshi ionlarga qarab o'zgaradi, ammo o'rtacha ortoperiodatlar bir oz deformatsiyalangan oktaedral geometriyani qabul qiladilar, rentgen nurlari diffraktsiyasi 1,89 Å.ga teng I-O bog'lanish uzunligini ko'rsatadi. [11][8] Metaperyodatlar o'rtacha I-O masofasi 1,78 Å bo'lgan buzilgan tetraedral geometriyani qabul qiladi. .[12][13]
Reaksiyalar
[tahrir | manbasini tahrirlash]Parchalanish reaksiyalari
[tahrir | manbasini tahrirlash]Peryodatlar turli xil 1,2-difunksiyali alkanlarda uglerod-uglerod aloqalarini uzishi mumkin.[14][15] Buning eng keng tarqalgan misoli diol bo'linishi bo'lib, u ham birinchi bo'lib kashf etilgan (Malaprade reaksiyasi).[16] Diollardan tashqari peryodatlar 1,2-gidroksi ketonlar, 1,2-diketonlar, a-keto kislotalar, a-gidroksi kislotalar, aminokislotalar,[17] 1,2-aminokislotalar,[18] 1,2 ni parchalashi mumkin. -diaminlar,[19] va epoksidlar[20] aldegidlar, ketonlar va karboksilik kislotalarni beradi.

Alkenlar, shuningdek, Lemieux-Jonson oksidlanishida oksidlanishi va parchalanishi mumkin. Bu osmiy tetroksidning katalitik yuklanishidan foydalanadi, bu periyod tomonidan in situ qayta tiklanadi. Umumiy jarayon ozonoliz jarayoniga teng.
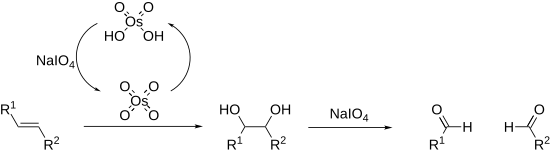
Ajralish reaksiyalari davriy efir deb ataladigan siklik oraliq mahsulot orqali boradi. Uning hosil bo'lishiga pH va harorat[21] ta'sir qilishi mumkin, lekin substratning geometriyasi eng kuchli ta'sir qiladi, sis -diollar trans -diollarga qaraganda sezilarli darajada tezroq reaksiyaga kirishadi.[22] Reaksiyalar ekzotermikdir va odatda 0 C° da amalga oshiriladi Peryodik tuzlar faqat suvda oson eriydi, chunki reaksiyalar odatda suvli muhitda amalga oshiriladi. Eruvchanlik muammosi bo'lsa, davriy kislota ishlatilishi mumkin, chunki u spirtlarda eriydi; fazali transfer katalizatorlari ikki fazali reaksiya aralashmalarida ham samarali. Haddan tashqari holatlarda peryodat xuddi shunday reaksiyaga kirishadigan va organik erituvchilarda eriydigan qo'rg'oshin tetraatsetatga almashtirilishi mumkin (Criegee oksidlanishi).

Peryodat parchalanishi ko'pincha molekulyar biokimyoda saxarid halqalarini o'zgartirish maqsadida qo'llaniladi, chunki ko'plab besh va olti a'zoli shakarlarda visinal diollar mavjud. Tarixiy jihatdan u monosaxaridlarning tuzilishini aniqlash uchun ham ishlatilgan.[23][24]
Qog'oz ishlab chiqarishda qo'llaniladigan dialdegid kraxmalini hosil qilish uchun peryodat parchalanishi sanoat miqyosida amalga oshirilishi mumkin.[25]
Oksidlanish reaksiyalari
[tahrir | manbasini tahrirlash]Peryodatlar kuchli oksidlovchi moddalardir. Ular katexolni 1,2-benzokinongacha va gidroxinonni 1,4-benzokinongacha oksidlashi mumkin.[26] Sulfidlar samarali ravishda sulfoksidlarga oksidlanishi mumkin.[27] Peryodatlar permanganat,[28] osmiy tetroksid[29] va ruteniy tetroksid kabi boshqa kuchli noorganik oksidlovchilarni hosil qilish uchun etarlicha kuchli.
Ishlatilishi
[tahrir | manbasini tahrirlash]Mikroskopda ishlatiladigan bir nechta bo'yash vositalari peryodatga asoslangan (masalan, davriy kislota-Schiff dog'i va Jons dog'i)
Peryodatlar, shuningdek, pirotexnikada foydalanish uchun oksidlovchi moddalar sifatida ishlatilgan.[30] 2013-yilda AQSh armiyasi atrof-muhitga zararli kimyoviy moddalar bo'lgan bariy nitrat va kaliy perkloratni o'z o'q-dorilarida foydalanish uchun natriy metaperiodat bilan almashtirishini e'lon qildi.[31]
Boshqa oksianionlar
[tahrir | manbasini tahrirlash]Peryodat yod -1, +1, +3, +5 yoki +7 oksidlanish darajalarini qabul qilishi mumkin bo'lgan bir qator oksianionlarning bir qismidir. Bir qator neytral yod oksidlari ham ma'lum.
| Yodning oksidlanish darajasi | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Ism | yodid | gipoiyodit | yodit | yod | davriy |
| Formula | men - | IO - | IO-</br> IO | IO-</br> IO | IO-</br> IO yoki IO5−</br> IO |
| Tuzilishi | </img> | </img> |
Yana qarang
[tahrir | manbasini tahrirlash]- Natriy periodat
- Kaliy periodat
- Davriy kislota
Manbalar
[tahrir | manbasini tahrirlash]- ↑ Ammermüller, F.; Magnus, G. (1833). „Ueber eine neue Verbindung des Jods mit Sauerstoff, die Ueberjodsäure“. Annalen der Physik und Chemie (German). 104-jild, № 7. 514–525-bet. Bibcode:1833AnP...104..514A. doi:10.1002/andp.18331040709.
{{cite magazine}}: CS1 maint: unrecognized language () - ↑ 2,0 2,1 Riley, edited by Georg Brauer; translated by Scripta Technica, Inc. Translation editor Reed F.. Handbook of preparative inorganic chemistry. Volume 1, 2nd, New York, N.Y.: Academic Press, 1963 — 323–324 bet. ISBN 012126601X. Manba xatosi: Invalid
<ref>tag; name "Brauer" defined multiple times with different content - ↑ Hill, Arthur E. (October 1928). „Ternary Systems. VII. The Periodates of the Alkali Metals“. Journal of the American Chemical Society. 50-jild, № 10. 2678–2692-bet. doi:10.1021/ja01397a013.
- ↑ Parsons, Roger. Handbook of electrochemical constants. Butterworths Scientific Publications Ltd, 1959 — 71 bet.
- ↑ Aylett, founded by A.F. Holleman; continued by Egon Wiberg; translated by Mary Eagleson, William Brewer; revised by Bernhard J.. Inorganic chemistry, 1st English ed., [edited] by Nils Wiberg., San Diego, Calif.: Berlin: Academic Press, W. de Gruyter., 2001 — 454 bet. ISBN 0123526515.
- ↑ Burgot, Jean-Louis. Ionic equilibria in analytical chemistry. New York: Springer, 30-mart 2012-yil — 358 bet. ISBN 978-1441983824.
- ↑ Ropp, Richard C.. Encyclopedia of the alkaline earth compounds. Oxford: Elsevier Science, 31-dekabr 2012-yil — 96 bet. ISBN 978-0444595539.
- ↑ 8,0 8,1 Feikema, Y. D. (1966). „The crystal structures of two oxy-acids of iodine. I. A study of orthoperiodic acid, H5IO6, by neutron diffraction“. Acta Crystallographica. 20-jild, № 6. 765–769-bet. doi:10.1107/S0365110X66001828. Manba xatosi: Invalid
<ref>tag; name "H5IO6" defined multiple times with different content - ↑ Greenwood, N.N.. Chemistry of the elements, 2nd, Oxford: Butterworth-Heinemann, 2006 — 874 bet. ISBN 0750633654.
- ↑ Ivanov, A.; Popov, A.; Boldyrev, A.; Zhdankin, V. (2014). „The I=X (X = O,N,C) Double Bond in Hypervalent Iodine Compounds: Is it Real?“. Angew. Chem. Int. Ed. 53-jild, № 36. 9617–9621-bet. doi:10.1002/anie.201405142. PMID 25045143.
- ↑ Tichý, K.; Rüegg, A.; Beneš, J. (1980). „Neutron diffraction study of diammonium trihydrogen periodate, (NH4)2H3IO6, and its deuterium analogue, (ND4)2D3IO6“. Acta Crystallographica Section B. 36-jild, № 5. 1028–1032-bet. doi:10.1107/S0567740880005225.
- ↑ Levason, W.; Webster, M. (15–iyun 1999–yil). „Ammonium tetraoxoiodate(VII)“. Acta Crystallographica Section C. 55-jild, № 6. IUC9900052-bet. doi:10.1107/S0108270199099394.
{{cite magazine}}: CS1 maint: date format () - ↑ Kálmán, A.; Cruickshank, D. W. J. (1970). „Refinement of the structure of NaIO4“. Acta Crystallographica Section B. 26-jild, № 11. 1782–1785-bet. doi:10.1107/S0567740870004880.
- ↑ Sklarz, B. (1967). „Organic chemistry of periodates“. Quarterly Reviews, Chemical Society. 21-jild, № 1. 3-bet. doi:10.1039/QR9672100003.
- ↑ Bamford, edited by C.H.. Reactions of non-metallic inorganic compounds. Amsterdam: Elsevier Pub. Co., 1972 — 435 bet. ISBN 9780080868011.
- ↑ L. Malaprade, Bull. Soc. Chim. Fr. 3, 1, 833 (1934)
- ↑ Clamp, J.R.; Hough, L. (Jan 1965). „The Periodate Oxidation of Amino Acids with Reference to Studies on Glycoproteins“. The Biochemical Journal. 94-jild. 17–24-bet. doi:10.1042/bj0940017. PMC 1206400. PMID 14342227.
- ↑ Nicolet, Ben H.; Shinn, Leo A. (June 1939). „THE ACTION OF PERIODIC ACID ON α-AMINO ALCOHOLS“. Journal of the American Chemical Society. 61-jild, № 6. 1615-bet. doi:10.1021/ja01875a521.
- ↑ Maros, László; Molnár-Perl, Ibolya; Schissel, Enikó; Szerdahelyi, Vilmos (1980). „Mechanism of the periodate oxidation of ethane-1,2-diamine, N,N′-dimethylethane-1,2-diamine, and 2-aminoethanol“. Journal of the Chemical Society, Perkin Transactions 2. № 1. 39–45-bet. doi:10.1039/P29800000039.
- ↑ Telvekar, Vikas N.; Patel, Dharmeshkumar J.; Mishra, Sanket J. (2008). „Oxidative Cleavage of Epoxides Using Aqueous Sodium Paraperiodate“. Synthetic Communications. 39-jild, № 2. 311–315-bet. doi:10.1080/00397910802372574.
- ↑ Buist, G. J.; Bunton, C. A.; Hipperson, W. C. P. (1971). „The mechanism of oxidation of α-glycols by periodic acid. Part X. The oxidation of pinacol, and a general discussion of the stability of periodate esters and their role in the mechanism of oxidation“. Journal of the Chemical Society B: Physical Organic. 2128–2142-bet. doi:10.1039/J29710002128.
- ↑ McMurry, John. Organic chemistry, 8th ed., [international ed.], Singapore: Brooks/Cole Cengage Learning, 2012 — 312 bet. ISBN 978-0840054531.
- ↑ Jackson, Ernest L.; Hudson, C. S. (June 1937). „Studies on the Cleavage of the Carbon Chain of Glycosides by Oxidation. A New Method for Determining Ring Structures and Alpha and Beta Configurations of Glycosides“. Journal of the American Chemical Society. 59-jild, № 6. 994–1003-bet. doi:10.1021/ja01285a010.
- ↑ Robyt, John F.. Essentials of carbohydrate chemistry. New York: Springer, 1998. ISBN 0387949518.
- ↑ Yu, Jiugao; Chang, Peter R.; Ma, Xiaofei (January 2010). „The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch“. Carbohydrate Polymers. 79-jild, № 2. 296–300-bet. doi:10.1016/j.carbpol.2009.08.005.
- ↑ Weidman, S. W.; Kaiser, E. T. (December 1966). „The Mechanism of the Periodate Oxidation of Aromatic Systems. III. A Kinetic Study of the Periodate Oxidation of Catechol“. Journal of the American Chemical Society. 88-jild, № 24. 5820–5827-bet. doi:10.1021/ja00976a024.
- ↑ Leonard, Nelson J.; Johnson, Carl R. (January 1962). „Periodate Oxidation of Sulfides to Sulfoxides. Scope of the Reaction“. The Journal of Organic Chemistry. 27-jild, № 1. 282–284-bet. doi:10.1021/jo01048a504.
- ↑ Lemieux, R. U.; Rudloff, E. Von (November 1955). „Periodate–Permanganate Oxidations: I. Oxidation of Olefins“. Canadian Journal of Chemistry. 33-jild, № 11. 1701–1709-bet. doi:10.1139/v55-208.
- ↑ Pappo, R.; Allen, Jr., D. S.; Lemieux, R. U.; Johnson, W. S. (1956). „Notes - Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds“. The Journal of Organic Chemistry. 21-jild, № 4. 478–479-bet. doi:10.1021/jo01110a606. ISSN 0022-3263.
- ↑ Moretti, Jared D.; Sabatini, Jesse J.; Chen, Gary (9–iyul 2012–yil). „Periodate Salts as Pyrotechnic Oxidizers: Development of Barium- and Perchlorate-Free Incendiary Formulations“. Angewandte Chemie International Edition. 51-jild, № 28. 6981–6983-bet. doi:10.1002/anie.201202589. PMID 22639415.
{{cite magazine}}: CS1 maint: date format () - ↑ „Picatinny to remove tons of toxins from lethal rounds“. U.S. Army. Qaraldi: 31-oktabr 2013-yil.



