Saraton immunoterapiyasi
Saraton immunoterapiyasi saraton kasalligini davolash uchun immunitet tizimini rag'batlantirish, immunitet tizimining kasallik bilan kurashish uchun tabiiy immunitetni yaxshilashdir. Bu saraton immunologiyasining fundamental tadqiqotlarini qo'llash va onkologiyaning o'sib borayotgan kichik ixtisosligi hisoblanadi.
Saraton immunoterapiyasi saraton hujayralarida ko'pincha o'simta antigenlariga ega ekanligidan foydalanadi, ularning yuzasida immun tizimining antitana oqsillari tomonidan aniqlanishi mumkin bo'lgan molekulalar, ular bilan bog'lanadi. O'simta antigeni ko'pincha oqsillar yoki boshqa makromolekulalardir ..Oddiy antitanalar tashqi patogenlar bilan bog'lanadi, ammo o'zgartirilgan immunoterapiya antitanalari immunitet tizimini inhibe qilish yoki o'ldirish uchun saraton hujayralarini belgilaydigan va aniqlaydigan o'simta antigenlariga bog'lanadi. Saraton immunoterapiyasining klinik muvaffaqiyati saratonning turli shakllari orasida juda o'zgaruvchan. Masalan; oshqozon saratonining ayrim kichik turlari yondashuvga yaxshi munosabatda bo'lishadi, boshqa kichik tiplar uchun immunoterapiya samarali emas[1].
Dendritik hujayra terapiyasi[tahrir | manbasini tahrirlash]

Giyohvand moddalar[tahrir | manbasini tahrirlash]
Sipuleucel-T 2010 yilda asemptomatik yoki minimal simptomatik metastatik kastratsiyaga chidamli prostata saratonini davolash uchun tasdiqlangan. Davolash antigen taqdim qiluvchi hujayralarni qondan leykaferez yo'li bilan olib tashlash va ularni GM-CSF va prostata beziga xos prostata kislotasi fosfatazasidan tayyorlangan PA2024 termoyadroviy oqsili bilan o'stirishdan iborat. Bu jarayon uch marta takrorlanadi[2][3][4][5].
Antitana terapiyasi[tahrir | manbasini tahrirlash]


FDA tomonidan tasdiqlangan antitanalar[tahrir | manbasini tahrirlash]
| Antikor | Brendning nomi | Turi | Maqsad | Tasdiqlash sanasi | Tasdiqlangan davolash(lar) |
|---|---|---|---|---|---|
| Alemtuzumab | Kampat | insoniylashtirilgan | CD52 | 2001 yil | B-hujayrali surunkali limfotsitik leykemiya (CLL) [8] |
| Atezolizumab | Tecentriq | insoniylashtirilgan | PD-L1 | 2016 yil | qovuq saratoni [9] |
| Avelumab | Bavensio | inson | PD-L1 | 2017 yil | metastatik Merkel hujayrali karsinoma [10] |
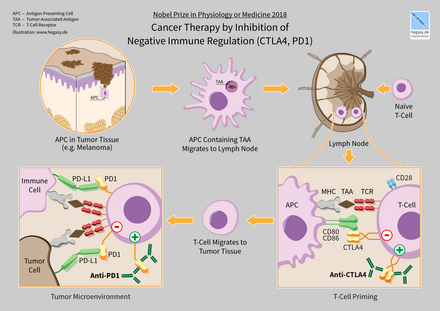
| Ipilimumab | Yervoy | inson | CTLA4 | 2011 yil | metastatik melanoma [11] |
| Elotuzumab | Empliciti | insoniylashtirilgan | SLAMF7 | 2015 yil | ko'p miyelom [12] |
| Ofatumumab | Arzerra | inson | CD20 | 2009 yil | refrakter CLL [13] |
| Nivolumab | Opdivo | inson | PD-1 | 2014 yil | rezektsiya qilinmaydigan yoki metastatik melanoma, skuamoz kichik hujayrali bo'lmagan o'pka saratoni, buyrak hujayrali karsinoma, yo'g'on ichak saratoni, gepatotsellyulyar karsinoma, klassik hodgkin lenfoma [14] [15] |
| Pembrolizumab | Keytruda | insoniylashtirilgan | PD-1 | 2014 yil | rezektsiya qilinmaydigan yoki metastatik melanoma, skuamoz kichik hujayrali bo'lmagan o'pka saratoni (NSCLC), [16] Xodgkin limfomasi, [17] Merkel hujayrali karsinoma (MCC), [18] asosiy mediastinal B-hujayrali limfoma (PMBCL), [19] oshqozon saraton, bachadon bo'yni saratoni [20] |
| Rituximab | Rituxan, Mabthera | kimerik | CD20 | 1997 yil | Hodgkin bo'lmagan lenfoma [21] |
| Durvalumab | Imfinzi | inson | PD-L1 | 2017 yil | qovuq saratoni [22] kichik hujayrali bo'lmagan o'pka saratoni [23] |
Qabul qiluvchi T-hujayrali terapiya[tahrir | manbasini tahrirlash]

Anti-GD2 antitanalari[tahrir | manbasini tahrirlash]

Yana qarang[tahrir | manbasini tahrirlash]
- Saratonga qarshi emlash
- Antigen 5T4
- Coley toksinlari
- Kombinativ ablasyon va immunoterapiya
- Krioimmunoterapiya
- Fotoimmunoterapiya
Ma'lumotnomalar[tahrir | manbasini tahrirlash]
Havolalar[tahrir | manbasini tahrirlash]
- "Saratonni davolash uchun immunoterapiya" mavzusidagi primer, NIH
- Immunoterapiya - saraton kasalligini davolash uchun immunitet tizimidan foydalanish (Wayback Machine saytida 2017-04-04 sanasida arxivlangan)
- Saraton tadqiqot instituti - saraton immunoterapiyasi nima
- ↑ "Targeting the Myeloid-Derived Suppressor Cell Compartment for Inducing Responsiveness to Immune Checkpoint Blockade Is Best Limited to Specific Subtypes of Gastric Cancers.". Gastroenterology 161 (2): 727. August 2021. doi:10.1053/j.gastro.2021.03.047. PMID 33798523.
- ↑ "Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer". Human Vaccines & Immunotherapeutics 8 (4): 534–39. April 2012. doi:10.4161/hv.19795. PMID 22832254.
- ↑ "Progress in emerging therapies for advanced prostate cancer". Cancer Treatment Reviews 39 (3): 275–89. May 2013. doi:10.1016/j.ctrv.2012.09.005. PMID 23107383.
- ↑ "Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate-resistant prostate cancer". Vaccine 30 (29): 4394–97. June 2012. doi:10.1016/j.vaccine.2011.11.058. PMID 22122856.
- ↑ "Building on sipuleucel-T for immunologic treatment of castration-resistant prostate cancer". Cancer Control 20 (1): 7–16. January 2013. doi:10.1177/107327481302000103. PMID 23302902.
- ↑ "Antibody therapy of cancer". Nature Reviews. Cancer 12 (4): 278–87. March 2012. doi:10.1038/nrc3236. PMID 22437872.
- ↑ "Immunotherapy: past, present and future". Nature Medicine 9 (3): 269–77. March 2003. doi:10.1038/nm0303-269. PMID 12612576. https://zenodo.org/record/1233435.
- ↑ "FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia". The Oncologist 13 (2): 167–74. February 2008. doi:10.1634/theoncologist.2007-0218. PMID 18305062.
- ↑ „FDA approves new, targeted treatment for bladder cancer“. FDA (2016-yil 18-may). Qaraldi: 2016-yil 20-may.
- ↑ „US Food and Drug Administration – Avelumab Prescribing Label“.
- ↑ „FDA approval for Ipilimumab“. 2015-yil 6-aprelda asl nusxadan arxivlangan. Qaraldi: 2013-yil 7-noyabr.
- ↑ „Bristol-Myers Squibb and AbbVie Receive U.S. FDA Breakthrough Therapy Designation for Elotuzumab, an Investigational Humanized Monoclonal Antibody for Multiple Myeloma | BMS Newsroom“.
- ↑ "U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab". Clinical Cancer Research 16 (17): 4331–38. September 2010. doi:10.1158/1078-0432.CCR-10-0570. PMID 20601446.
- ↑ "The future of immune checkpoint therapy". Science 348 (6230): 56–61. April 2015. doi:10.1126/science.aaa8172. PMID 25838373.
- ↑ „Opdivo (nivolumab) FDA Approval History“. Drugs.com.
- ↑ "FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC". FDA. 20 December 2019. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm624659.htm.
- ↑ "Pembrolizumab (KEYTRUDA) for classical Hodgkin lymphoma". FDA. 9 February 2019. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm546893.htm.
- ↑ "FDA approves pembrolizumab for Merkel cell carcinoma". FDA. 20 December 2019. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm628867.htm.
- ↑ "FDA approves pembrolizumab for treatment of relapsed or refractory PMBCL". FDA. 9 February 2019. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610670.htm.
- ↑ „National Cancer Institute - Pembrolizumab Use in Cancer“ (2014-yil 18-sentyabr).
- ↑ "FDA approves new kind of lymphoma treatment. Food and Drug Administration". AIDS Treatment News (284): 2–3. December 1997. PMID 11364912.
- ↑ Center for Drug Evaluation and Research. „Approved Drugs – Durvalumab (Imfinzi)“. fda.gov. Qaraldi: 2017-yil 6-may.
- ↑ "FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC". FDA. 9 February 2019. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm597248.htm.
