Melatonin
Melatonin o'simlik va hayvonlarda mavjud bo'lgan tabiiy mahsulotdir. U asosan hayvonlarda tunda miyadagi pineal bez tomonidan chiqariladigan gormon sifatida tanilgan va uzoq vaqt davomida uyqu-uyg'onish siklini nazorat qilish bilan bog'liq[1][2].
Umurtqali hayvonlarda melatonin sirkadiyalik ritmlarni sinxronlashtirishda, shu jumladan uyqu-uyg'onish vaqtini va qon bosimini tartibga solishda va mavsumiy ritmiklikni nazorat qilishda, shu jumladan ko'payish, semizlik, mo'rtlashish va qish uyqusida ishtirok etadi[3]. Uning ko'p ta'siri melatonin retseptorlarini faollashtirish orqali, boshqalari esa antioksidant roli bilan bog'liq. O'simliklarda u oksidlovchi stressdan himoya qilish vazifasini bajaradi. U turli xil ovqatlarda ham mavjud[4].
Biologik faollik
[tahrir | manbasini tahrirlash]Odamlarda melatonin melatonin retseptorlari 1 (pikomolyar bog'lanish yaqinligi) va melatonin retseptorlari 2 (nanomolyar bog'lanish yaqinligi) ning to'liq agonisti bo'lib, ikkalasi ham G-oqsil bilan bog'langan retseptorlari (GPCR) sinfiga tegishli. Melatonin retseptorlari 1 va 2 ikkalasi ham Gi/o-bog'langan GPCR hisoblanadi, garchi melatonin 1 retseptorlari ham Gq bilan bog'langan. Melatonin, shuningdek, mitoxondriya ichidagi yuqori sig'imli erkin radikallarni tozalash vositasi sifatida ishlaydi, shuningdek, melatonin retseptorlari orqali signal o'tkazish orqali superoksid dismutaza, glutation peroksidaza, glutation reduktaza va katalaza kabi antioksidant fermentlarning ifodalanishiga yordam beradi[5][6][7][8][9][10].
Biologik funksiyalar
[tahrir | manbasini tahrirlash]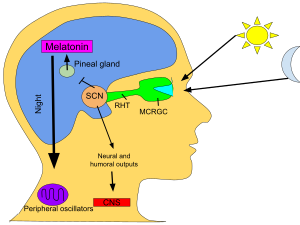
Antioksidant
[tahrir | manbasini tahrirlash]Melatonin birinchi marta kuchli antioksidant va erkin radikallarni tozalash vositasi sifatida 1993-yilda xabar qilingan. In vitroda melatonin kislorod radikallarini, shu jumladan OH•, O2−• va reaktiv azot turlarini NO• kabi toʻgʻridan-toʻgʻri tozalovchi vazifasini bajaradi. O'simliklarda melatonin har bir antioksidantning umumiy samaradorligini oshirish uchun boshqa antioksidantlar bilan ishlaydi. Melatonin E vitaminiga qaraganda ikki baravar faol ekanligi isbotlangan, u eng samarali lipofil antioksidant hisoblanadi. Melatonin retseptorlari orqali signal o'tkazish melatonin superoksid dismutaza, glutation peroksidaza, glutation reduktaza va katalaza kabi antioksidant fermentlarning ifodalanishiga yordam beradi[5][6].
Immun tizimi
[tahrir | manbasini tahrirlash]Melatonin immunitet tizimi bilan o'zaro ta'sir qilishi ma'lum bo'lsa-da, bu o'zaro ta'sirlarning tafsilotlari aniq emas. Yallig'lanishga qarshi ta'sir eng dolzarb bo'lib tuyuladi. Kasallikni davolashda melatoninning samaradorligini baholash uchun bir nechta sinovlar o'tkazildi. Mavjud ma'lumotlarning aksariyati kichik, to'liq bo'lmagan sinovlarga asoslangan. Har qanday ijobiy immunologik ta'sir immunokompetent hujayralarda ifodalangan yuqori afinitetli retseptorlarga (MT1 va MT2) ta'sir qiluvchi melatoninning natijasi deb hisoblanadi. Preklinik tadqiqotlarda melatonin sitokin ishlab chiqarishni kuchaytirishi mumkin va bu orqali orttirilgan immunitet tanqisligiga qarshi kurashadi. Ba'zi tadqiqotlar shuni ko'rsatadiki, melatonin yuqumli kasalliklarga, shu jumladan OIV va bakterial infektsiyalarga qarshi kurashda va saraton kasalligini davolashda foydali bo'lishi mumkin[11][12].
Og'irlikni tartibga solish
[tahrir | manbasini tahrirlash]Melatoninning kilogramm ortishini boshqarishi mumkin bo'lgan mexanizm uning leptinga inhibitiv ta'siridir. Leptin inson organizmidagi energiya holatining uzoq muddatli ko'rsatkichi sifatida ishlaydi. Uyg'onish vaqtidan tashqarida leptin ta'sirini bostirish orqali melatonin leptin qarshiligini yumshatib, kunduzi leptin sezgirligini tiklashga yordam beradi[13][14].
Biokimyo
[tahrir | manbasini tahrirlash]Biosintez
[tahrir | manbasini tahrirlash]
Hayvonlarda melatoninning biosintezi gidroksillanish, dekarboksillanish, atsetillanish va L-triptofan bilan boshlangan metillanish orqali sodir bo'ladi. L-triptofan xorizmatdan shikimat yo'lida ishlab chiqariladi yoki oqsil katabolizmidan olinadi. Birinchi L-triptofan 5-gidroksitriptofan hosil qilish uchun triptofan gidroksilaz tomonidan indol halqasida gidroksillanadi. Ushbu oraliq mahsulot (5-HTP) serotonin ishlab chiqarish uchun piridoksal fosfat va 5-gidroksitriptofan dekarboksilaza tomonidan dekarboksillanadi.
Mexanizm
[tahrir | manbasini tahrirlash]
L-triptofanni gidroksillash uchun kofaktor tetrahidrobiopterin (THB) birinchi navbatda kislorod va triptofan gidroksilazaning faol joyi temir bilan reaksiyaga kirishishi kerak. Ushbu mexanizm yaxshi tushunilmagan, ammo ikkita mexanizm taklif qilingan:
1. Bir elektronning THB dan O2 ga sekin oʻtishi THB radikali bilan rekombinatsiyalanib, 4a-peroksipterin hosil qilishi mumkin boʻlgan superoksid hosil qilishi mumkin. Keyin 4a-peroksipterin faol temir (II) bilan reaksiyaga kirishib, temir-peroksipterin oraliq mahsulotini hosil qilishi yoki kislorod atomini to'g'ridan-to'g'ri temirga o'tkazishi mumkin.
2. O2 birinchi navbatda faol joy temir (II) bilan reaksiyaga kirishib, temir (III) superoksidini ishlab chiqarishi mumkin, keyinchalik u THB bilan reaksiyaga kirishib, temir-peroksipterin oraliq mahsulotini hosil qilishi mumkin.
Temir-peroksipterin oraliq mahsulotidan olingan temir (IV) oksidi indol halqasining C5 pozitsiyasida karbokatiya hosil qilish uchun qo'sh bog' bilan tanlab hujumga uchraydi. Vodorodning 1,2 siljishi va keyin C5 dagi ikkita vodorod atomidan birining yo'qolishi 5-gidroksi-L-triptofanni berish uchun aromatiklikni tiklaydi[15].
N-asetilserotonin gidroksil holatida S-adenozil metionin (SAM) tomonidan metillanadi va S-adenozil homosistein (SAH) va melatonin hosil bo'ladi[16][17].
Tibbiy foydalanish
[tahrir | manbasini tahrirlash]Melatonin uyqusizlik va sirkadiyalik ritm buzilishi kabi uyqu buzilishlarini davolashda dori sifatida ishlatiladi, masalan, kechikkan uyqu fazasi buzilishi, jet lag buzilishi va smenali ish buzilishi[18]. Tibbiyotda melatonindan tashqari, ramelteon, tasimelteon va agomelatin kabi sintetik melatonin retseptorlari agonistlari ham qo'llaniladi[19][20].
Oziq-ovqat qo'shimchasi sifatida
[tahrir | manbasini tahrirlash]Melatonin rejalashtirilgan dori bo'lmagan mamlakatlarda parhez qo'shimchasi sifatida sotiladi. AQShda melatonin oziqaviy qo'shimcha hisoblanadi va rejalashtirilgan yoki faqat retsept bo'yicha beriladigan dori-darmonlarga nisbatan qattiqroq FDA qoidalaridan ozod qilinadi. 2017-yilda AQSh bozorida so'ralgan melatonin qo'shimchalarining 70 foizi reklama qilingan melatonin tarkibining <10 foizini o'z ichiga olgan[21]. Ba'zi qo'shimchalar reklama qilingan melatonin miqdorining >400% ni o'z ichiga olgan[21].
There is evidence to suggest that the typical advertised dose of most melatonin supplements (>1mg) are excessive for the treatment of insomnia and may even be detrimental to overall sleep quality.[22][23] 4mg of controlled release melatonin has been found to cause excessive melatonin levels upon waking (>50pg/ml), possibly contributing to lethargy in the morning.[22] By contrast,.4mg of controlled release melatonin was not found to cause elevated melatonin levels upon waking.[22]
Tarixi
[tahrir | manbasini tahrirlash]Melatonin birinchi bo'lib ba'zi amfibiyalar va sudraluvchilar teri rangini o'zgartirish mexanizmi bilan bog'liq holda topilgan[24][25]. 1917-yildayoq Keri Pratt Makkord va Floyd P. Allen sigirlarning pineal bezlarini boqish ekstrakti qoramtir epidermal melanoforlarni qisqartirish orqali kurtak terisini oqartirishini aniqladilar[26][27].
In 1958, dermatology professor Aaron B. Lerner and colleagues at Yale University, in the hope that a substance from the pineal might be useful in treating skin diseases, isolated the hormone from bovine pineal gland extracts and named it melatonin.[28] In the mid-70s Lynch et al. demonstrated that the production of melatonin exhibits a circadian rhythm in human pineal glands.[29]
Boshqa turlarda
[tahrir | manbasini tahrirlash]Boshqa jonzotlar
[tahrir | manbasini tahrirlash]Umurtqali hayvonlarda melatonin qorong'uda, odatda tunda, miyaning markazida joylashgan, ammo qon-miya to'sig'idan tashqarida joylashgan kichik ichki sekretsiya bezi - pineal bez tomonidan ishlab chiqariladi. Yorug'lik/qorong'i ma'lumotlar melatonin signalidan ko'ra (bir paytlar taxmin qilinganidek) ko'zning to'r pardasining fotosensitiv ganglion hujayralaridan supraxiazmatik yadrolarga etib boradi. "Qorong'ulik gormoni" deb nomlanuvchi melatoninning oqshom chog'ida paydo bo'lishi tungi (tungi faol) hayvonlarning faolligini va kunduzgi hayvonlarning, shu jumladan odamlarning uxlashiga yordam beradi[30].
Kechasi melatonin leptin darajasini tartibga solib, uning darajasini pasaytiradi.
Ketasianlar melatonin sintezi uchun barcha genlarni, shuningdek, melatonin retseptorlari uchun genlarni yo'qotdilar.[31] Bu ularning bir yarim sharik uyqu rejimi (bir vaqtning o'zida bitta miya yarim shari) bilan bog'liq deb taxmin qilinadi. Xuddi shunday tendentsiyalar sireniyaliklarda ham topilgan[31].
Plants
[tahrir | manbasini tahrirlash]1987-yilda o'simliklarda aniqlanmagunga qadar, melatonin o'nlab yillar davomida asosan hayvonlarning neyrogormoni deb hisoblangan. 1970-yillarda qahva ekstraktida melatonin aniqlanganda, u ekstraksiya jarayonining yon mahsuloti ekanligiga ishonishgan. Biroq, keyinchalik, melatonin tekshirilgan barcha o'simliklarda topilgan. U o'simliklarning barcha turli qismlarida, jumladan, barglari, poyalari, ildizlari, mevalari va urug'larida turli nisbatlarda mavjud[32][33]. Melatonin kontsentratsiyasi nafaqat o'simlik turlari, balki agrotexnik o'sish sharoitlariga qarab bir xil turdagi navlar orasida ham farqlanadi, pikogramlardan grammiga bir necha mikrogramgacha o'zgaradi[34][35]. Qahva, choy, vino va pivo kabi mashhur ichimliklar hamda makkajo'xori, guruch, bug'doy, arpa va jo'xori kabi ekinlarda yuqori melatonin kontsentratsiyasi aniqlangan. Ba'zi keng tarqalgan oziq-ovqat va ichimliklar, jumladan, qahva va yong'oqda melatonin kontsentratsiyasi qondagi melatonin darajasini kunduzgi boshlang'ich qiymatlardan oshirish uchun etarli darajada yuqori ekanligi taxmin qilingan yoki o'lchangan.
Melatoninning o'simlik gormoni sifatidagi roli aniq aniqlanmagan bo'lsa-da, uning o'sish va fotosintez kabi jarayonlarda ishtirok etishi yaxshi tasdiqlangan. Ba'zi o'simlik turlarida melatonin darajasidagi endogen sirkadiyalik ritmlarning faqat cheklangan dalillari ko'rsatilgan va hayvonlarda ma'lum bo'lganlarga o'xshash membrana bilan bog'langan retseptorlari tasvirlanmagan. Aksincha, melatonin o'simliklarda o'sish regulyatori, shuningdek, ekologik stressdan himoya qiluvchi muhim rol o'ynaydi. U o'simliklarda biologik stresslarga, masalan, qo'ziqorin infektsiyasiga va haroratning haddan tashqari o'zgarishi, toksinlar, tuproq sho'rlanishining oshishi, qurg'oqchilik va boshqalar kabi biologik bo'lmagan stresslarga duchor bo'lganda sintezlanadi[34][36][37].
Gerbitsidlar keltirib chiqaradigan oksidlovchi stress in vivo jonli ravishda yuqori melatoninli transgenik guruchda yumshatilgan[38][39].
Qo'ziqorin kasalliklariga chidamlilik boshqa rol o'ynaydi. Qo'shilgan melatonin Malus prunifolia ning Diplocarpon mali ga qarshi chidamliligini oshiradi[39][40]. Bundan tashqari, Alternaria, Botrytis va Fusarium spp kabi qo'ziqorin patogenlarida o'sish inhibitori sifatida ishlaydi. INFEKTSION tezligini pasaytiradi. Urug'larni davolash sifatida Lupinus albusni qo'ziqorinlardan himoya qiladi. Pseudomonas syringae pomidorini sezilarli darajada sekinlashtiradi DC3000 Arabidopsis thaliana va Nicotiana benthamiana yuqtiradi[40].
Zamburug'lar
[tahrir | manbasini tahrirlash]Melatonin o'simlik-patogen tizimlarida Phytophthora infestansda stressga chidamlilikni kamaytirishi kuzatilgan[41].
Hodisa
[tahrir | manbasini tahrirlash]Oziq-ovqat mahsulotlari
[tahrir | manbasini tahrirlash]Tabiiy melatonin 0,17–13,46 ng/g gacha bo'lgan tort gilos[42], banan, olxo'ri, uzum, guruch, don, o'tlar, zaytun moyi[43], sharob[44] va pivoda[45] tabiiy ravishda uchraydi[46]. Sut va gilos iste'mol qilish uyqu sifatini yaxshilashi mumkin. Qushlar guruch kabi melatoninga boy o'simlik ozuqalarini iste'mol qilganda, melatonin ularning miyasidagi melatonin retseptorlari bilan bog'lanadi. Odamlar banan, ananas va apelsin kabi melatoninga boy oziq-ovqatlarni iste'mol qilganda, qonda melatonin darajasi sezilarli darajada oshadi[47].
Manbalar
[tahrir | manbasini tahrirlash]- ↑ Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL (avgust 2017). „Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders“ (PDF). Sleep Medicine Reviews. 34-jild. 10–22-bet. doi:10.1016/j.smrv.2016.06.005. hdl:20.500.11820/0e890bda-4b1d-4786-a907-a03b1580fd07. PMID 28648359.
- ↑ ADHD: Non-Pharmacologic Interventions, An Issue of Child and Adolescent Psychiatric Clinics of North America, E-Book. Elsevier Health Sciences, 2014 — 888 bet. ISBN 978-0-323-32602-5.
- ↑ Altun A, Ugur-Altun B (may 2007). „Melatonin: therapeutic and clinical utilization“. International Journal of Clinical Practice. 61-jild, № 5. 835–45-bet. doi:10.1111/j.1742-1241.2006.01191.x. PMID 17298593. S2CID 18050554.
- ↑ „Melatonin: Side Effects, Uses, Dosage (Kids/Adults)“. Drugs.com. Qaraldi: 2019-yil 9-yanvar.
- ↑ 5,0 5,1 Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A (2017). „Melatonin and human mitochondrial diseases“. Journal of Research in Medical Sciences. 22-jild. 2-bet. doi:10.4103/1735-1995.199092. PMC 5361446. PMID 28400824.
- ↑ 6,0 6,1 Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. (sentyabr 2016). „Update on melatonin receptors: IUPHAR Review 20“. British Journal of Pharmacology. 173-jild, № 18. 2702–25-bet. doi:10.1111/bph.13536. PMC 4995287. PMID 27314810. „Hence, one melatonin molecule and its associated metabolites could scavenge a large number of reactive species, and thus, the overall antioxidant capacity of melatonin is believed to be greater than that of other well‐known antioxidants, such as vitamin C and vitamin E, under in vitro or in vivo conditions (Gitto et al., 2001; Sharma and Haldar, 2006; Ortiz et al., 2013).“
- ↑ Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (Noyabr 2017). „Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas“. Cellular and Molecular Life Sciences. 74-jild, № 21. 3863–3881-bet. doi:10.1007/s00018-017-2609-7. PMID 28864909. S2CID 23820389. „melatonin is specifically targeted to the mitochondria where it seems to function as an apex antioxidant ... The measurement of the subcellular distribution of melatonin has shown that the concentration of this indole in the mitochondria greatly exceeds that in the blood.“
- ↑ Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (oktabr 2016). „Melatonin as an antioxidant: under promises but over delivers“. Journal of Pineal Research. 61-jild, № 3. 253–78-bet. doi:10.1111/jpi.12360. PMID 27500468. S2CID 35435683. „There is credible evidence to suggest that melatonin should be classified as a mitochondria-targeted antioxidant.“
- ↑ Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, et al. (Noyabr 2015). „Melatonin: an ancient molecule that makes oxygen metabolically tolerable“. Journal of Pineal Research. 59-jild, № 4. 403–19-bet. doi:10.1111/jpi.12267. PMID 26272235. S2CID 24373303. „While originally thought to be produced exclusively in and secreted from the vertebrate pineal gland [53], it is now known that the indole is present in many, perhaps all, vertebrate organs [54] and in organs of all plants that have been investigated [48, 55, 56]. That melatonin is not relegated solely to the pineal gland is also emphasized by the reports that it is present in invertebrates [57–59], which lack a pineal gland and some of which consist of only a single cell.“
- ↑ Mayo JC, Sainz RM, González-Menéndez P, Hevia D, Cernuda-Cernuda R (Noyabr 2017). „Melatonin transport into mitochondria“. Cellular and Molecular Life Sciences. 74-jild, № 21. 3927–3940-bet. doi:10.1007/s00018-017-2616-8. PMID 28828619. S2CID 10920415.
- ↑ Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (may 2006). „The modulatory role of melatonin on immune responsiveness“. Current Opinion in Investigational Drugs. 7-jild, № 5. 423–31-bet. PMID 16729718.
- ↑ Maestroni GJ (Mart 2001). „The immunotherapeutic potential of melatonin“. Expert Opinion on Investigational Drugs. 10-jild, № 3. 467–76-bet. doi:10.1517/13543784.10.3.467. PMID 11227046. S2CID 6822594.
- ↑ Suriagandhi V, Nachiappan V (Yanvar 2022). „Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance“. Behavioural Brain Research. 417-jild. 113598-bet. doi:10.1016/j.bbr.2021.113598. PMID 34563600.
- ↑ Buonfiglio D, Parthimos R, Dantas R, Cerqueira Silva R, Gomes G, Andrade-Silva J, et al. (2018). „Melatonin Absence Leads to Long-Term Leptin Resistance and Overweight in Rats“. Frontiers in Endocrinology. 9-jild. 122-bet. doi:10.3389/fendo.2018.00122. PMC 5881424. PMID 29636725.
- ↑ Roberts KM, Fitzpatrick PF (Aprel 2013). „Mechanisms of tryptophan and tyrosine hydroxylase“. IUBMB Life. 65-jild, № 4. 350–7-bet. doi:10.1002/iub.1144. PMC 4270200. PMID 23441081.
- ↑ Medicinal Natural Products. A Biosynthetic Approach, 2nd, Wiley, 2002. ISBN 978-0-471-49640-3.
- ↑ Donohue SJ, Roseboom PH, Illnerova H, Weller JL, Klein DC (oktabr 1993). „Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA“. DNA and Cell Biology. 12-jild, № 8. 715–27-bet. doi:10.1089/dna.1993.12.715. PMID 8397829.
- ↑ Riha RL (Noyabr 2018). „The use and misuse of exogenous melatonin in the treatment of sleep disorders“. Curr Opin Pulm Med. 24-jild, № 6. 543–548-bet. doi:10.1097/MCP.0000000000000522. PMID 30148726.
- ↑ Williams WP, McLin DE, Dressman MA, Neubauer DN (sentyabr 2016). „Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders“. Pharmacotherapy. 36-jild, № 9. 1028–41-bet. doi:10.1002/phar.1822. PMC 5108473. PMID 27500861.
- ↑ Atkin T, Comai S, Gobbi G (Aprel 2018). „Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery“. Pharmacol Rev. 70-jild, № 2. 197–245-bet. doi:10.1124/pr.117.014381. PMID 29487083.
- ↑ 21,0 21,1 Grigg-Damberger MM, Ianakieva D (Fevral 2017). „Poor Quality Control of Over-the-Counter Melatonin: What They Say Is Often Not What You Get“. Journal of Clinical Sleep Medicine. 13-jild, № 2. 163–165-bet. doi:10.5664/jcsm.6434. PMC 5263069. PMID 28095978.
- ↑ 22,0 22,1 22,2 Gooneratne NS, Edwards AY, Zhou C, Cuellar N, Grandner MA, Barrett JS (may 2012). „Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults“. Journal of Pineal Research. 52-jild, № 4. 437–445-bet. doi:10.1111/j.1600-079X.2011.00958.x. PMC 3682489. PMID 22348451.
- ↑ Li J, Somers VK, Xu H, Lopez-Jimenez F, Covassin N (Fevral 2022). „Trends in Use of Melatonin Supplements Among US Adults, 1999-2018“. Jama. 327-jild, № 5. 483–485-bet. doi:10.1001/jama.2021.23652. PMID 35103775.
- ↑ Filadelfi AM, Castrucci AM (may 1996). „Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates“. Journal of Pineal Research. 20-jild, № 4. 175–86-bet. doi:10.1111/j.1600-079X.1996.tb00256.x. PMID 8836950. S2CID 41959214.
- ↑ Sugden D, Davidson K, Hough KA, Teh MT (oktabr 2004). „Melatonin, melatonin receptors and melanophores: a moving story“. Pigment Cell Research. 17-jild, № 5. 454–60-bet. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- ↑ Encyclopedia of dietary supplements. New York, N.Y: Marcel Dekker, 2005 — 457–66 bet. ISBN 978-0-8247-5504-1.
- ↑ McCord CP, Allen FP (Yanvar 1917). „Evidences associating pineal gland function with alterations in pigmentation“. J Exp Zool. 23-jild, № 1. 206–24-bet. doi:10.1002/jez.1400230108.
- ↑ Lerner AB, Case JD, Takahashi Y (iyul 1960). „Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands“. The Journal of Biological Chemistry. 235-jild, № 7. 1992–7-bet. doi:10.1016/S0021-9258(18)69351-2. PMID 14415935.
- ↑ Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH (Yanvar 1975). „Daily rhythm in human urinary melatonin“. Science. 187-jild, № 4172. 169–71-bet. Bibcode:1975Sci...187..169L. doi:10.1126/science.1167425. PMID 1167425.
- ↑ Foster RG (Iyun 2020). „Sleep, circadian rhythms and health“. Interface Focus. 10-jild, № 3. 20190098-bet. doi:10.1098/rsfs.2019.0098. PMC 7202392. PMID 32382406.
- ↑ 31,0 31,1 Huelsmann M, Hecker N, Springer MS, Gatesy J, Sharma V, Hiller M (sentyabr 2019). „Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations“. Science Advances. 5-jild, № 9. eaaw6671-bet. Bibcode:2019SciA....5.6671H. doi:10.1126/sciadv.aaw6671. PMC 6760925. PMID 31579821.
- ↑ Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (Yanvar 2012). „Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science“. Journal of Experimental Botany. 63-jild, № 2. 577–97-bet. doi:10.1093/jxb/err256. PMID 22016420.
- ↑ Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (1 Yanvar 2009). „Phytomelatonin: a review“. Journal of Experimental Botany. 60-jild, № 1. 57–69-bet. doi:10.1093/jxb/ern284. PMID 19033551. S2CID 15738948.
- ↑ 34,0 34,1 Hardeland R (Fevral 2015). „Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions“. Journal of Experimental Botany. 66-jild, № 3. 627–46-bet. doi:10.1093/jxb/eru386. PMID 25240067.
- ↑ Bonnefont-Rousselot D, Collin F (Noyabr 2010). „Melatonin: action as antioxidant and potential applications in human disease and aging“. Toxicology. 278-jild, № 1. 55–67-bet. doi:10.1016/j.tox.2010.04.008. PMID 20417677.
- ↑ Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A (Aprel 2015). „Phytomelatonin: assisting plants to survive and thrive“. Molecules. 20-jild, № 4. 7396–437-bet. doi:10.3390/molecules20047396. PMC 6272735. PMID 25911967.
- ↑ Arnao MB, Hernández-Ruiz J (sentyabr 2015). „Functions of melatonin in plants: a review“. Journal of Pineal Research. 59-jild, № 2. 133–50-bet. doi:10.1111/jpi.12253. PMID 26094813.
- ↑ Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (Aprel 2013). „Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress“. Journal of Pineal Research. 54-jild, № 3. Wiley. 258–63-bet. doi:10.1111/j.1600-079x.2012.01029.x. PMID 22856683. S2CID 6291664.
- ↑ 39,0 39,1 Arnao MB, Hernández-Ruiz J (dekabr 2014). „Melatonin: plant growth regulator and/or biostimulator during stress?“. Trends in Plant Science. 19-jild, № 12. Elsevier. 789–97-bet. doi:10.1016/j.tplants.2014.07.006. PMID 25156541. S2CID 38637203.
- ↑ 40,0 40,1 Arnao MB, Hernández-Ruiz J (sentyabr 2015). „Functions of melatonin in plants: a review“. Journal of Pineal Research. 59-jild, № 2. Wiley. 133–50-bet. doi:10.1111/jpi.12253. PMID 26094813. S2CID 19887642.
- ↑ Socaciu AI, Ionuţ R, Socaciu MA, Ungur AP, Bârsan M, Chiorean A, et al. (dekabr 2020). „Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases“. Reviews in Endocrine & Metabolic Disorders. 21-jild, № 4. 465–478-bet. doi:10.1007/s11154-020-09570-9. PMID 32691289. S2CID 220657247.
- ↑ Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (oktabr 2001). „Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus)“. Journal of Agricultural and Food Chemistry. 49-jild, № 10. 4898–902-bet. doi:10.1021/jf010321. PMID 11600041.
- ↑ González-Flores D, Velardo B, Garrido M, González-Gómez D, Lozano M, Ayuso MC, Barriga C, Paredes SD, Rodríguez AB (2011). „Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content“. Journal of Food and Nutrition Research. 50-jild, № 4. 229–36-bet.
- ↑ Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S (may 2011). „Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection“. Journal of Pineal Research. 50-jild, № 4. 374–80-bet. doi:10.1111/j.1600-079X.2010.00853.x. PMID 21342247. S2CID 8034935.
- ↑ Salehi B (5 iyul 2019). „Melatonin in Medicinal and Food Plants“ (PDF). Cells. 681-jild. 29 Noyabr 2021da asl nusxadan (PDF) arxivlandi. Qaraldi: 21 sentyabr 2022.
- ↑ Pereira N, Naufel MF, Ribeiro EB, Tufik S, Hachul H (Yanvar 2020). „Influence of Dietary Sources of Melatonin on Sleep Quality: A Review“. Journal of Food Science. 85-jild, № 1. Wiley. 5–13-bet. doi:10.1111/1750-3841.14952. PMID 31856339.
- ↑ Sae-Teaw M, Johns J, Johns NP, Subongkot S (avgust 2013). „Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers“. Journal of Pineal Research. 55-jild, № 1. 58–64-bet. doi:10.1111/jpi.12025. PMID 23137025. S2CID 979886.
Tashqi havola
[tahrir | manbasini tahrirlash]| Vikiomborda Melatonin haqida turkum mavjud |
- „Melatonin“. Drug Information Portal. U.S. National Library of Medicine.
- „Melatonin“. Chemwatch.

