Korrannulen
Korannulen - kimyoviy formulasi C20H10[1] bo'lgan polisiklik aromatik uglevodorod.Molekula 5 ta benzol halqasi bilan birlashtirilgan siklopentan halqasidan iborat, shuning uchun uning boshqa nomi sirlendir. Bu ilmiy qiziqish uyg'otadi, chunki u geodezik poliarendir va bukminsterfullerenning bir bo'lagi sifatida qabul qilinishi mumkin. Ushbu bog'liqlik va uning piyola shakli tufayli korannulen, shuningdek, bukkiboul sifatida ham tanilgan. Korannulen −64°C.[2] da 10,2 kkal/mol (42,7 kJ/mol) inversiya toʻsigʻi bilan piyola-piyola inversiyasini koʻrsatadi.
Sintez[tahrir | manbasini tahrirlash]
Korannulenni sintez qilishda bir nechta sintetik yo'llar mavjud. Fleshli vakuumli piroliz usullari odatda eritma-kimyo sinteziga qaraganda pastroq kimyoviy rentabellikka ega, ammo ko'proq hosilalarni olish yo'llarini taklif qiladi. Korannulan birinchi marta 1966-yilda ko'p bosqichli organik sintez orqali ajratilgan.[3] 1971-yilda korannulanning sintezi va xossalari haqida xabar berilgan.[4] Fleshli vakuumli piroliz usuli 1991-yilda qo'llanilgan[5] Eritmalar kimyosiga asoslangan sintezlardan biri[6] nukleofil joy almashish – oktabromidni natriy gidroksid bilan yo'q qilish reaksiyasidan iborat:
Brom o'rnini bosuvchi moddalar ortiqcha n -butillitiy bilan chiqariladi.
Korannulenning kilogramm miqyosda sinteziga erishildi.[7]
Korannulen halqasini etinil guruhlari,[2][8][9] efir guruhlari,[10] tioefir guruhlari,[11] platina funktsional guruhlari,[12] aril guruhlari,[13] fenalenil birlashtirilgan[14] va indeno kengaytmalari.[15] va ferrosin guruhlari orqali boyitildi.[16]
Xushbo'ylik[tahrir | manbasini tahrirlash]
Ushbu birikma uchun kuzatilgan aromatiklik annulen ichidagi annulen modeli bilan izohlanadi. Ushbu modelga ko'ra, korannulen aromatik 14 elektronli annulenil kation bilan o'ralgan aromatik 6 elektronli siklopentadienil anionidan iborat. Bu model Bart va Lotton tomonidan 1966-yilda korannulenning birinchi sintezida taklif qilingan[3] Ular, shuningdek, annulen ichidagi annulen modelidan olingan "korannulen" arzimas nomini taklif qilishdi: yadro + annulen.
Biroq, keyingi nazariy hisob-kitoblar bu taxminning to'g'riligini isbotladi.[17][18]
Reaksiyalar[tahrir | manbasini tahrirlash]
Qisqartirish[tahrir | manbasini tahrirlash]
Korannulen bir elektronli qisqarishlar seriyasida tetraaniongacha kamayishi mumkin. Bu ishqoriy metallar, elektrokimyoviy va asoslar bilan amalga oshirildi. Korannulen dianioni antiaromatik, tetraanion esa yana aromatikdir. Qaytaruvchi vosita sifatida litiy bilan ikkita tetraanion supramolekulyar dimer hosil qiladi, ular bir -biriga 4 ta litiy ionlari o'rtasida va 2 juft yuqorida va pastda joylashgan ikkita idishdan iborat.[19] Ushbu o'z-o'zini yig'ish motivi fullerenlarni tashkil qilishda qo'llanilgan. Beshta elektron bilan zaryadlangan penta almashtirilgan fullerenlar (metil yoki fenil guruhlari bilan) interstitsial litiy kationlari bilan "tikilgan" qo'shimcha korannulen tetraanion kosasi bilan supramolekulyar dimerlarni hosil qiladi.[20] Tegishli tizimda 5 ta litiy ioni ikkita korannulen kosasi orasiga biriktirilgan[21]
Bitta siklopentakorannulenda tetraanionlarni bog'laydigan 2 C–Li–C bog'lari bo'lgan NMR spektroskopiyasida konkav - botiq agregat kuzatiladi.[22]
Metalllar annulenning qavariq yuziga bog'lanishga moyil. Seziy / toj efir tizimi uchun konkav bog'lanishi haqida xabar berilgan[23]
Oksidlanish[tahrir | manbasini tahrirlash]
UV 193-nm fotoionizatsiyasi korannulen radikal kationini hosil qiluvchi aromatik elektronlar tarmog'ida joylashgan ikki marta degeneratsiyalangan E1-HOMO dan p-elektronni samarali ravishda olib tashlaydi.[24] HOMO orbitalidagi degeneratsiya tufayli korannulen radikal kationi o'zining dastlabki C5v molekulyar tuzilishida beqaror va shuning uchun Jahn-Teller (JT) tebranish buzilishiga duchor bo'ladi.
Elektrosprey ionizatsiyasidan foydalanib, protonlangan korannulen kationi ishlab chiqarildi, unda protonlanish joyi periferik sp2 - uglerod atomida joylashganligi kuzatildi.[24]
Elektrofillar bilan reaksiya[tahrir | manbasini tahrirlash]
Korannulen elektrofiller bilan reaksiyaga kirishib, korannulen karbokatini hosil qilishi mumkin. Xlorometan va alyuminiy xlorid bilan reaksiyaga kirishish natijasida markazda joylashgan metil guruhi bilan AlCl4 - tuzi kation markazi chetida joylashgan. X-nurlari diffraktsiya tahlili shuni ko'rsatadiki, yangi uglerod-uglerod bog'i cho'zilgan (157 pm)[25]
Bikorannulenil[tahrir | manbasini tahrirlash]
Bikorannulenil korannulenning degidrogen birikmasi mahsulotidir. C20H9-C20H9 formulasi bilan u bitta C-C aloqasi orqali bog'langan ikkita korannulen birligidan iborat. Molekulaning stereokimyosi ikkita chiral elementdan iborat: yakka almashtirilgan korannulenilning assimetriyasi va markaziy bog'lanish atrofidagi spiral burilish. Neytral holatda bikorannulenil 12 ta konformator sifatida mavjud bo'lib, ular bir nechta piyola inversiyasi va bog'lanish aylanishlari orqali o'zaro aylanadi.[26] Bikorannulenil kaliy metalli bilan dianionga aylantirilganda, markaziy bog'lanish sezilarli qo'sh bog'lanish xarakterini oladi. Ushbu o'zgarish markaziy bog'lanishda lokalizatsiya qilingan LUMO orbitaliga ega bo'lgan orbital strukturaga bog'liq.[27] Bikorannulenil litiy metall bilan oktanionga qaytarilganda, u o'z-o'zidan supramolekulyar oligomerlarga aylanadi.[28] Ushbu motiv "zaryadlangan poliaren stacking" ni tasvirlaydi.
Tadqiqot[tahrir | manbasini tahrirlash]
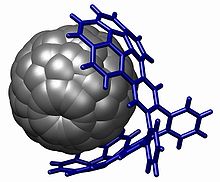
Korannulen guruhi mezbon-mehmon kimyosida pi stackingga asoslangan o'zaro ta'sirlarda, xususan fullerenlar (buckycatcher)[29][30] bilan, shuningdek, nitrobenzol[31] bilan qo'llaniladi.
Alkil bilan almashtirilgan korannulenlar termotropik olti burchakli ustunli suyuq kristalli mezofazani hosil qiladi.[32] Korannulen dendrimerda asosiy guruh sifatida ham ishlatilgan.[13] Boshqa PAHlar singari, korannulen metallarni bog'laydi.[33][34][35][36][37][38][39] Etinil guruhlari bo'lgan korannulenlar ularning ko'k emitentlar sifatida ishlatilishi uchun tekshiriladi.[9] Struktura infraqizil spektroskopiya, Raman spektroskopiyasi va rentgen fotoelektron spektroskopiyasi yordamida tahlil qilindi.[40]
Yana qarang[tahrir | manbasini tahrirlash]
- Koronen
- Helitsen
- Geodezik poliaren
Manbalar[tahrir | manbasini tahrirlash]
- ↑ Scott, L. T.; Bronstein, H. E.; Preda, D. V.; Ansems, R. B. M.; Bratcher, M. S.; Hagen, S. (1999). „Geodesic polyarenes with exposed concave surfaces“. Pure and Applied Chemistry. 71-jild, № 2. 209-bet. doi:10.1351/pac199971020209.
- ↑ 2,0 2,1 Scott, L. T.; Hashemi, M. M.; Bratcher, M. S. (1992). „Corannulene bowl-to-bowl inversion is rapid at room temperature“. Journal of the American Chemical Society. 114-jild, № 5. 1920–1921-bet. doi:10.1021/ja00031a079.
- ↑ 3,0 3,1 Barth, W. E.; Lawton, R. G. (1966). „Dibenzo[ghi,mno]fluoranthene“. Journal of the American Chemical Society. 88-jild, № 2. 380–381-bet. doi:10.1021/ja00954a049.
- ↑ Lawton, Richard G.; Barth, Wayne E. (April 1971). „Synthesis of corannulene“. Journal of the American Chemical Society. 93-jild, № 7. 1730–1745-bet. doi:10.1021/ja00736a028.
- ↑ Scott, L. T.; Hashemi, M. M.; Meyer, D. T.; Warren, H. B. (1991). „Corannulene. A convenient new synthesis“. Journal of the American Chemical Society. 113-jild, № 18. 7082–7084-bet. doi:10.1021/ja00018a082.
- ↑ Sygula, A.; Rabideau, P. W. (2000). „A Practical, Large Scale Synthesis of the Corannulene System“. Journal of the American Chemical Society. 122-jild, № 26. 6323–6324-bet. doi:10.1021/ja0011461.
- ↑ Butterfield, A.; Gilomen, B.; Siegel, J. (2012). „Kilogram-Scale Production of Corannulene“. Organic Process Research & Development. 16-jild, № 4. 664–676-bet. doi:10.1021/op200387s.
- ↑ Wu, Y.; Bandera, D.; Maag, R.; Linden, A.; Baldridge, K.; Siegel, J. (2008). „Multiethynyl corannulenes: synthesis, structure, and properties“. Journal of the American Chemical Society. 130-jild, № 32. 10729–10739-bet. doi:10.1021/ja802334n. PMID 18642812.
- ↑ 9,0 9,1 Mack, J.; Vogel, P.; Jones, D.; Kaval, N.; Sutton, A. (2007). „The development of corannulene-based blue emitters“. Organic & Biomolecular Chemistry. 5-jild, № 15. 2448–2452-bet. doi:10.1039/b705621d. PMID 17637965. Manba xatosi: Invalid
<ref>tag; name "Mack" defined multiple times with different content - ↑ Gershoni-Poranne, R.; Pappo, D.; Solel, E.; Keinan, E. (2009). „Corannulene ethers via Ullmann condensation“. Organic Letters. 11-jild, № 22. 5146–5149-bet. doi:10.1021/ol902352k. PMID 19905024.
- ↑ Baldridge, K.; Hardcastle, K.; Seiders, T.; Siegel, J. (2010). „Synthesis, structure and properties of decakis(phenylthio)corannulene“. Organic & Biomolecular Chemistry. 8-jild, № 1. 53–55-bet. doi:10.1039/b919616a. PMID 20024131.
- ↑ Choi, H.; Kim, C.; Park, K. M.; Kim, J.; Kang, Y.; Ko, J. (2009). „Synthesis and structure of penta-platinum σ-bonded derivatives of corannulene“. Journal of Organometallic Chemistry. 694-jild, № 22. 3529–3532-bet. doi:10.1016/j.jorganchem.2009.07.015.
- ↑ 13,0 13,1 Pappo, D.; Mejuch, T.; Reany, O.; Solel, E.; Gurram, M.; Keinan, E. (2009). „Diverse Functionalization of Corannulene: Easy Access to Pentagonal Superstructure“. Organic Letters. 11-jild, № 5. 1063–1066-bet. doi:10.1021/ol8028127. PMID 19193048. Manba xatosi: Invalid
<ref>tag; name "pappo" defined multiple times with different content - ↑ Nishida, S.; Morita, Y.; Ueda, A.; Kobayashi, T.; Fukui, K.; Ogasawara, K.; Sato, K.; Takui, T.; Nakasuji, K. (2008). „Curve-structured phenalenyl chemistry: synthesis, electronic structure, and bowl-inversion barrier of a phenalenyl-fused corannulene anion“. Journal of the American Chemical Society. 130-jild, № 45. 14954–14955-bet. doi:10.1021/ja806708j. PMID 18937470.
- ↑ Steinberg, B.; Jackson, E.; Filatov, A.; Wakamiya, A.; Petrukhina, M.; Scott, L. (2009). „Aromatic pi-systems more curved than C(60). The complete family of all indenocorannulenes synthesized by iterative microwave-assisted intramolecular arylations“. Journal of the American Chemical Society. 131-jild, № 30. 10537–10545-bet. doi:10.1021/ja9031852. PMID 19722628.
- ↑ Topolinski, Berit; Schmidt, Bernd M.; Kathan, Michael; Troyanov, Sergej I.; Lentz, Dieter (2012). „Corannulenylferrocenes: towards a 1D, non-covalent metal–organic nanowire“. Chem. Commun. 48-jild, № 50. 6298–6300-bet. doi:10.1039/C2CC32275G. PMID 22595996.
- ↑ Sygula, A.; Rabideau, P. W. (1995). „Structure and inversion barriers of corannulene, its dianion and tetraanion. An ab initio study“. Journal of Molecular Structure: THEOCHEM. 333-jild, № 3. 215–226-bet. doi:10.1016/0166-1280(94)03961-J.
- ↑ Monaco, G.; Scott, L.; Zanasi, R. (2008). „Magnetic euripi in corannulene“. The Journal of Physical Chemistry A. 112-jild, № 35. 8136–8147-bet. Bibcode:2008JPCA..112.8136M. doi:10.1021/jp8038779. PMID 18693706.
- ↑ Ayalon, A.; Sygula, A.; Cheng, P.; Rabinovitz, M.; Rabideau, P.; Scott, L. (1994). „Stable High-Order Molecular Sandwiches: Hydrocarbon Polyanion Pairs with Multiple Lithium Ions Inside and out“. Science. 265-jild, № 5175. 1065–1067-bet. Bibcode:1994Sci...265.1065A. doi:10.1126/science.265.5175.1065. PMID 17832895.
- ↑ Aprahamian, I.; Eisenberg, D.; Hoffman, R.; Sternfeld, T.; Matsuo, Y.; Jackson, E.; Nakamura, E.; Scott, L.; Sheradsky, T. (2005). „Ball-and-socket stacking of supercharged geodesic polyarenes: bonding by interstitial lithium ions“. Journal of the American Chemical Society. 127-jild, № 26. 9581–9587-bet. doi:10.1021/ja0515102. PMID 15984885.
- ↑ Zabula, A. V. (2011). „A Main Group Metal Sandwich: Five Lithium Cations Jammed Between Two Corannulene Tetraanion Decks“. Science. 333-jild, № 6045. 1008–1011-bet. Bibcode:2011Sci...333.1008Z. doi:10.1126/science.1208686. PMID 21852497.
- ↑ Aprahamian, I.; Preda, D.; Bancu, M.; Belanger, A.; Sheradsky, T.; Scott, L.; Rabinovitz, M. (2006). „Reduction of bowl-shaped hydrocarbons: dianions and tetraanions of annelated corannulenes“. The Journal of Organic Chemistry. 71-jild, № 1. 290–298-bet. doi:10.1021/jo051949c. PMID 16388648.
- ↑ Spisak, S. N.; Zabula, A. V.; Filatov, A. S.; Rogachev, A. Y.; Petrukhina, M. A. (2011). „Selective Endo and Exo Binding of Alkali Metals to Corannulene“. Angewandte Chemie International Edition. 50-jild, № 35. 8090–8094-bet. doi:10.1002/anie.201103028. PMID 21748832.
- ↑ 24,0 24,1 Galué, Héctor Alvaro; Rice, Corey A.; Steill, Jeffrey D.; Oomens, Jos (1–yanvar 2011–yil). „Infrared spectroscopy of ionized corannulene in the gas phase“ (PDF). The Journal of Chemical Physics. 134-jild, № 5. 054310-bet. Bibcode:2011JChPh.134e4310G. doi:10.1063/1.3540661. PMID 21303123.
{{cite magazine}}: CS1 maint: date format () - ↑ Zabula, A. V.; Spisak, S. N.; Filatov, A. S.; Rogachev, A. Y.; Petrukhina, M. A. (2011). „A Strain-Releasing Trap for Highly Reactive Electrophiles: Structural Characterization of Bowl-Shaped Arenium Carbocations“. Angewandte Chemie International Edition. 50-jild, № 13. 2971–2974-bet. doi:10.1002/anie.201007762. PMID 21404379.
- ↑ Eisenberg, D.; Filatov, A.; Jackson, E.; Rabinovitz, M.; Petrukhina, M.; Scott, L.; Shenhar, R. (2008). „Bicorannulenyl: stereochemistry of a C40H18 biaryl composed of two chiral bowls“. The Journal of Organic Chemistry. 73-jild, № 16. 6073–6078-bet. doi:10.1021/jo800359z. PMID 18505292.
- ↑ Eisenberg, D.; Quimby, J. M.; Jackson, E. A.; Scott, L. T.; Shenhar, R. (2010). „The Bicorannulenyl Dianion: A Charged Overcrowded Ethylene“. Angewandte Chemie International Edition. 49-jild, № 41. 7538–7542-bet. doi:10.1002/anie.201002515. PMID 20814993.
- ↑ Eisenberg, D.; Quimby, J. M.; Jackson, E. A.; Scott, L. T.; Shenhar, R. (2010). „Highly Charged Supramolecular Oligomers Based on the Dimerization of Corannulene Tetraanion“. Chemical Communications. 46-jild, № 47. 9010–9012-bet. doi:10.1039/c0cc03965a. PMID 21057679.
- ↑ Sygula, A.; Fronczek, F.; Sygula, R.; Rabideau, P.; Olmstead, M. (2007). „A double concave hydrocarbon buckycatcher“. Journal of the American Chemical Society. 129-jild, № 13. 3842–3843-bet. doi:10.1021/ja070616p. PMID 17348661.
- ↑ Wong, B. M. (2009). „Noncovalent interactions in supramolecular complexes: a study on corannulene and the double concave buckycatcher“. Journal of Computational Chemistry. 30-jild, № 1. 51–56-bet. arXiv:1004.4243. doi:10.1002/jcc.21022. PMID 18504779.
- ↑ Kobryn, L.; Henry, W. P.; Fronczek, F. R.; Sygula, R.; Sygula, A. (2009). „Molecular clips and tweezers with corannulene pincers“. Tetrahedron Letters. 50-jild, № 51. 7124–7127-bet. doi:10.1016/j.tetlet.2009.09.177.
- ↑ Miyajima, D.; Tashiro, K.; Araoka, F.; Takezoe, H.; Kim, J.; Kato, K.; Takata, M.; Aida, T. (2009). „Liquid crystalline corannulene responsive to electric field“. Journal of the American Chemical Society. 131-jild, № 1. 44–45-bet. doi:10.1021/ja808396b. PMID 19128171.
- ↑ Seiders, T. Jon; Baldridge, Kim K.; O'Connor, Joseph M.; Siegel, Jay S. (1997). „Hexahapto Metal Coordination to Curved Polyaromatic Hydrocarbon Surfaces: The First Transition Metal Corannulene Complex“. J. Am. Chem. Soc. 119-jild, № 20. 4781–4782-bet. doi:10.1021/ja964380t.
- ↑ Siegel, Jay S.; Baldridge, Kim K.; Linden, Anthony; Dorta, Reto (2006). „d8 Rhodium and Iridium Complexes of Corannulene“. J. Am. Chem. Soc. 128-jild, № 33. 10644–10645-bet. doi:10.1021/ja062110x. PMID 16910635.
- ↑ Petrukhina, M. A. (2008). „Coordination of buckybowls: the first concave-bound metal complex“. Angewandte Chemie International Edition in English. 47-jild, № 9. 1550–1552-bet. doi:10.1002/anie.200704783. PMID 18214869.
- ↑ Zhu, B.; Ellern, A.; Sygula, A.; Sygula, R.; Angelici, R. J. (2007). „η6-Coordination of the Curved Carbon Surface of Corannulene (C20H10) to (η6-arene)M2+(M = Ru, Os)“. Organometallics. 26-jild, № 7. 1721–1728-bet. doi:10.1021/om0610795.
- ↑ Petrukhina, M. A.; Sevryugina, Y.; Rogachev, A. Y.; Jackson, E. A.; Scott, L. T. (2006). „Corannulene: A Preference forexo-Metal Binding. X-ray Structural Characterization of [Ru2(O2CCF3)2(CO)4·(η2-C20H10)2]“. Organometallics. 25-jild, № 22. 5492–5495-bet. doi:10.1021/om060350f.
- ↑ Siegel, J.; Baldridge, K.; Linden, A.; Dorta, R. (2006). „D8 rhodium and iridium complexes of corannulene“. Journal of the American Chemical Society. 128-jild, № 33. 10644–10645-bet. doi:10.1021/ja062110x. PMID 16910635.
- ↑ Bandera, D.; Baldridge, K. K.; Linden, A.; Dorta, R.; Siegel, J. S. (2011). „Stereoselective Coordination of C5-Symmetric Corannulene Derivatives with an Enantiomerically Pure [RhI(nbd*)] Metal Complex“. Angewandte Chemie International Edition. 50-jild, № 4. 865–867-bet. doi:10.1002/anie.201006877. PMID 21246679.
- ↑ Diana, Nooramalina; Yamada, Yasuhiro; Gohda, Syun; Ono, Hironobu; Kubo, Shingo; Sato, Satoshi (2021-02-01). „Carbon materials with high pentagon density“. Journal of Materials Science (inglizcha). 56-jild, № 4. 2912–2943-bet. Bibcode:2021JMatS..56.2912D. doi:10.1007/s10853-020-05392-x. ISSN 1573-4803.

![Siklopenta[bc]korannulen](http://upload.wikimedia.org/wikipedia/commons/thumb/2/21/Cyclopenta-bc-corannulene.png/100px-Cyclopenta-bc-corannulene.png)